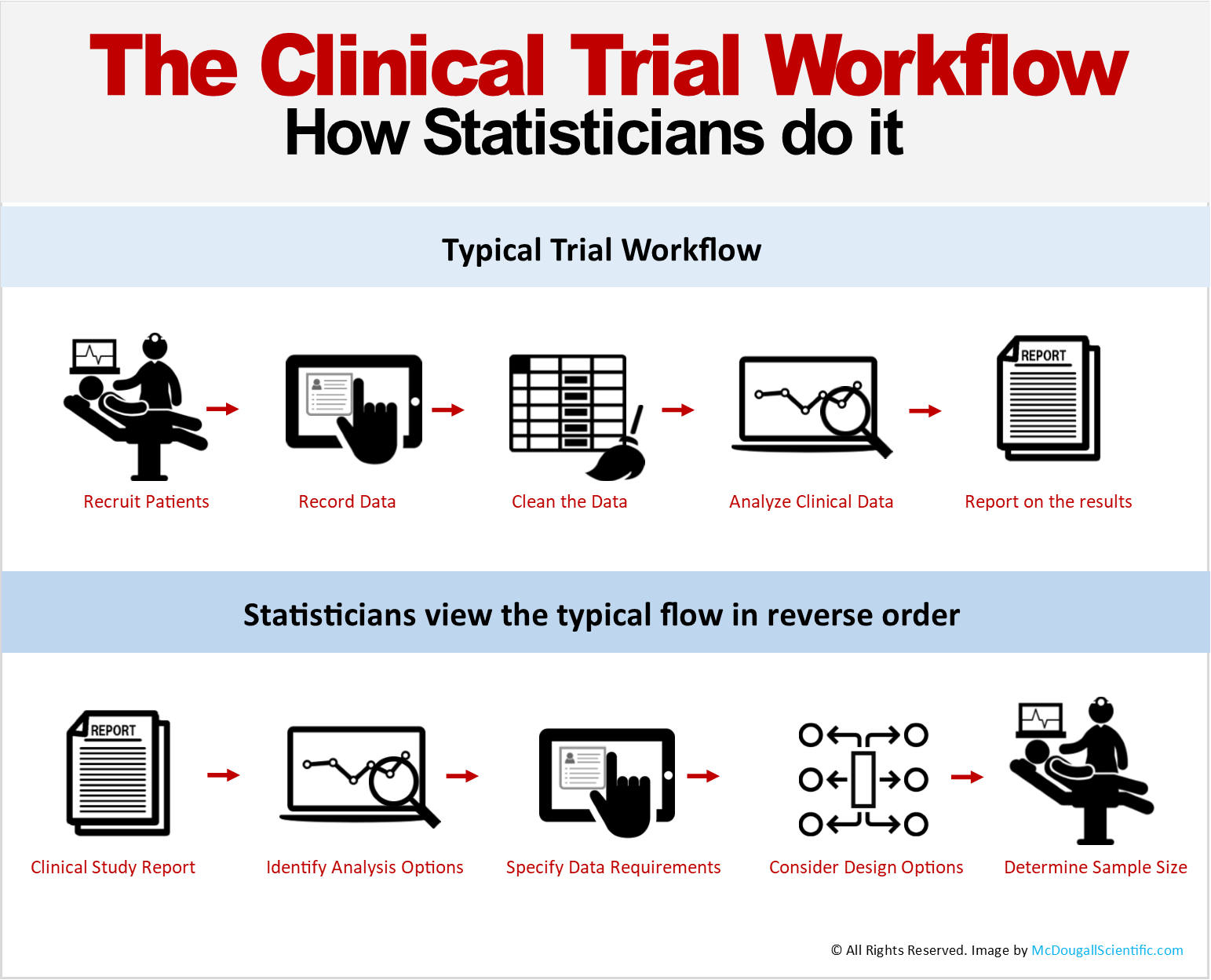
Design and Conduct of Clinical Trials
Table Of Content
- Avoiding designing failed clinical trials: main issues that lead to invalid data
- Types of Trial Designs
- Choosing the appropriate phase design
- Design and Interpretation of Clinical Trials
- Clinical trial phases: pharmacological studies
- Case‐control studies based within a defined cohort
- Failed (invalid) clinical trials vs. negative (but valid) clinical trials

Below, we created a framework based on the risk that the trial will fail vs. the chance that results will be beneficial to help the investigator choose the appropriate phase design. This week, we explore different types of trial designs, including parallel, crossover, group allocation, factorial, large simple, equivalency, non-inferiority, and adaptive designs. (i) Switch back design (ABA vs BAB arms) – Drug A → Drug B → Drug A in one arm versus Drug B → Drug A → Drug B in other arm. The switch back and multiple switchback designs are of emerging relevance with the advent of biosimilars where switchability and interchangeability of a biosimilar to a bio-originator molecule can only be confirmed by such trial designs.
Avoiding designing failed clinical trials: main issues that lead to invalid data
The advantage of this design is its flexibility such that it can be continued until a definitive conclusion can be reached for the particular subject being studied. The utility also rests in analyzing treatments that elicit heterogenous responses in different subjects. Data from many N-of 1 subjects can be even combined to derive population effect sizes by meta-analysis or Bayesian methods. Common clinical trial designs include single-arm trials, placebo-controlled trials, crossover trials, factorial trials, noninferiority trials, and designs for validating a diagnostic device. The choice of the structural design depends on the specific research questions of interest, characteristics of the disease and therapy, the endpoints, the availability of a control group, and on the availability of funding. Structural designs are discussed in an accompanying article in this special issue.
Types of Trial Designs
Therefore modern medicine has seen great advances as trial design and quality improved over the past 60 years, leading to better internal validity, efficiency and reporting (3). In this design, subjects are randomized to one or more study arms and each study arm will be allocated a different intervention. After randomization each participant will stay in their assigned treatment arm for the duration of the study [Figure 5].
Choosing the appropriate phase design
Phase II trials aim to further understand the safety and efficacy of an intervention to help decide whether or not to proceed to a phase III trial. Given the role of Phase II trials in determining the go/no-go decision to proceed for further testing in large confirmatory Phase III trials, it is crucial to select an appropriate endpoint, particularly in Phase II trials. Phase II endpoints should ideally be a strong surrogate for the Phase III endpoints (Yin et al., 2018). The delivery of an intervention whether drug, a dietary change, a lifestyle change, or a psychological therapy session counts as an intervention and hence must be dealt as a clinical trial [Figure 1].
Design and Interpretation of Clinical Trials

Subsequently the non-PASI 75 achievers are dropped from the trial due to lack of efficacy. PASI 75 responders are continued on the drug or are assigned to placebo and retention of PASI 75 response at 1 year after randomization can be compared between two arms. If there is no statistically significant difference in outcomes between the arms, an expensive biological can be administered till PASI 75 is achieved and then omitted, thereby reducing cost of therapy. Illustrative example – A comparative trial of Acitretin and Apremilast in palmoplantar psoriasis, where there exits clinical equipoise as regarding efficacy can be conducted as a randomized controlled (acitretin as active control) parallel arm trial design. This design is usually utilized to determine pharmacokinetic properties of a new drug (Phase 1 trials). Uncontrolled trials are known to produce greater mean effect estimates than a controlled trial, thereby inflating the expectations from the intervention.
Hookipa Pharma Announces Major HB-200 Clinical Trial Design - TipRanks.com - TipRanks
Hookipa Pharma Announces Major HB-200 Clinical Trial Design - TipRanks.com.
Posted: Thu, 25 Apr 2024 11:29:52 GMT [source]
Clinical trial phases: pharmacological studies
When both potential benefits and risks are high, we recommend running pilot, phase I, or small mechanistic phase II studies to better evaluate and quantify the risks. But researchers need to be careful as investigations in this category can fail more often than in less risky trials. One example is a phase III trial investigating tDCS for neuropathic pain that was not based on strong preliminary data. Indeed, this trial failed and thus patients were unnecessarily exposed to risk (47).
3 Randomization and stratification
This atypical presentation is usually described as case reports which provides a detailed and comprehensive description of the case.4 It is one of the earliest forms of research and provides an opportunity for the investigator to describe the observations that make a case unique. There are no inferences obtained and therefore cannot be generalized to the population which is a limitation. Most often than not, a series of case reports make a case series which is an atypical presentation found in a group of patients. This in turn poses the question for a new disease entity and further queries the investigator to look into mechanistic investigative opportunities to further explore.
Case‐control studies based within a defined cohort
Furthermore, Bolognini et al. evaluated the effects of 5-day transcranial direct current stimulation (tDCS) on the motor cortex of 8 amputees with PLP, based on the theory of maladaptive plasticity (10). These trials found promising effects of neuromodulation to alleviate PLP (low risk approach with high potential benefits). Other pilot studies concerned the application of sympathetic blocks (24) and cryoablation (12) for the treatment of PLP.
Failed (invalid) clinical trials vs. negative (but valid) clinical trials
Otherwise, the amount of preliminary data necessary will depend on the trial phase. For instance, in the early phases of a tDCS trial, the investigators tested the device in 8 PLP subjects with one session only, looking for immediate effects and testing different parameters (10). This, combined with further data on brain stimulation in pain, led the same group to then design a large pivotal trial using optimized parameters (25). We will also cover the gold standard for analysis of clinical trials, which is including all the participants in the analysis regardless of their actual treatment.
Having “double-blind” in the title of a trial does not imply that blinding was successful. Reviews of blinded trials suggest that many trials experience issues that jeopardize the blind. For example in a study assessing zinc for the treatment of the common cold(Prasad et al 2000) the blinding failed because the taste and aftertaste of zinc was distinctive. For example, OHARA and the ACTG are developing a study to evaluate the use of gentian violet (GV) for the treatment of oral candidiasis. GV has staining potential which could jeopardize the blind when the assessors conduct oral examinations after treatment.
However, in a case series, the cases are not compared to subjects without the manifestations and therefore it cannot determine which factors in the description are unique to the new disease entity. In summary, a benefit-risk assessment is always necessary when designing a trial, and the benefits and risks to research subjects vs. those for the target population are crucial to identify. Using a Bayesian analytic framework, between 40 and 125 patients are enrolled for each therapeutic arm, with pre-specified criteria for graduation (that is, declaring a therapy to be likely efficacious) or futility.
We know this subject lived ≥5 years and this can be used to evaluate and estimate survival up to 5 years. After 5 years the subject cannot contribute data for estimating or quantifying survival and they are termed censored. Different statistical analyses are needed for time-to-event versus quantitative data to account for censoring. Clinicians and other decision makers in healthcare use results from clinical trials to inform practice.
Once the rationale for a trial has been established, selection of the outcome(s) of interest is essential. Examples of health outcomes include quality of life, symptoms, adverse events, and patient-reported outcomes. Treatment outcomes include assessing safety or efficacy of the intervention; examples include tumor shrinkage, hematologic outcomes, intermediate or surrogate outcomes, time to event outcomes (e.g. overall survival or progression-free survival), and surgical outcomes. It is common to have one or two primary outcomes, and one or two secondary outcomes.
Randomization can be balanced where both groups are of equal size or unbalanced where groups are of unequal size. Finally, when confounding factors may be of concern, stratification may be considered as an additional design component. Although randomization aims to reduce confounding by making treatment groups as similar as possible except for the treatment assigned, it is nevertheless possible for the groups to differ with respect to some important factors. Examples of such factors include gender and age, and other factors specific to the study context. To avoid this possibility, identify these potential confounding factors and include stratification as part of the randomization process.
Most drugs studied (about 90%) never make it to market, as human trials do not show efficacy (27–29). Attrition is highly costly and frustrating, yet such studies help by showing us what does not work – “validation using known failures” (32) – assuming they are published. Clinical trials investigate new therapeutic interventions in a controlled environment, aiming to minimize biases and build on truth (1). Although experimentation has marked progress throughout human history, James Lind’s “A treatise of the scurvy” published in 1753 pioneered the methodology of modern controlled trials (1,2). Further progress on statistical and other methodological techniques (such as better blinding methods) together with improved ethical guidelines helped advance the science of clinical trial methodology.
Comments
Post a Comment