Clinical Trial Designs PMC
Table Of Content
CIT can broadcast your seminar, conference or meeting live to a world-wide audience over the Internet as a real-time streaming video. The event can be recorded and made available for viewers to watch at their convenience as an on-demand video or a downloadable file. Some of the considerations here are shared under the more general topic of design of experiments but there can be others, in particular related to patient confidentiality and ethics. Some of the authors have been involved in a number of the trials referenced in this paper.
The argument framework is a flexible approach to evidence in healthcare
Navigating the Challenges of Clinical Trial Design - BioSpace
Navigating the Challenges of Clinical Trial Design.
Posted: Mon, 06 Nov 2023 08:00:00 GMT [source]
Researchers could then consider using a sham injection that induces a similar reaction. Variation can also be reduced with standardization of the manner in which study participants are treated and evaluated via training. For example, in studies that involve imaging, it is very important to have an imaging protocol that standardizes the manner in which images are collected to reduce added variation due to inconsistent patient positioning. Training modules can be developed to instruct site personnel on the appropriate administration of evaluations. We have discussed various clinical research study designs in this comprehensive review.
Seasonal studies
Finally, we will review the essential ethical consideration involved in conducting experiments on people. When blinding is implemented in a clinical trial, a plan for assessing the effectiveness of the blinding may be arranged. This usually requires two blinding questionnaires, one completed by the trial participant and the other completed by the local investigator or person that conducts the evaluation of the trial participant.
Build your subject-matter expertise
A randomized clinical trial represents a controlled experiment in which the investigator applies an intervention to a group of patients and subsequently observes its effect on 1 or more outcomes over time. In this article, we outline the key elements that need to be considered during the design phase of a surgical clinical trial (Box). An active control is an active intervention that has often shown effectiveness to treat the disease under study. Often an active control is selected because it is the standard of care (SOC) treatment for the disease under study.
Repurposing a diabetes drug to treat Parkinson’s disease
(a) The disease must be chronic, stable, and incurable and characteristics must not vary for the duration of the two study periods and the interim wash out period and (b) the effect of each drug must not be irreversible. Bioequivalence and biosimilar equivalence studies usually utilize a cross over design. The duration of follow-up for the patient is longer than for a parallel design, and there is a risk that a significant number of patients do not complete the study and drop out leading to compromised study power. Treatment effects are efficiently isolated by controlling for bias and confounding and by minimizing variation.
The primary outcome should be directly related to the mechanism of action of the intervention, clinically meaningful, relevant to the patient, clearly defined, and measurable. These principles highlight the importance of the design process being collaborative, not only among clinicians and statisticians, but also including patient advocates, patients and their caregivers. Friedman et al. (2010) and Wu and Sargent (2010) offer more considerations on choosing endpoints. Well-designed clinical trials are essential for the advancement of modern medicine. Developing an in-depth understanding of existing preliminary data and a risk-benefit analysis can lead to an optimal research question and a solid trial design.
Newer Study Designs
Digital clinical trial startup to lay off half of its U.S. workforce by summer - Crain's New York Business
Digital clinical trial startup to lay off half of its U.S. workforce by summer.
Posted: Thu, 25 Apr 2024 09:30:00 GMT [source]
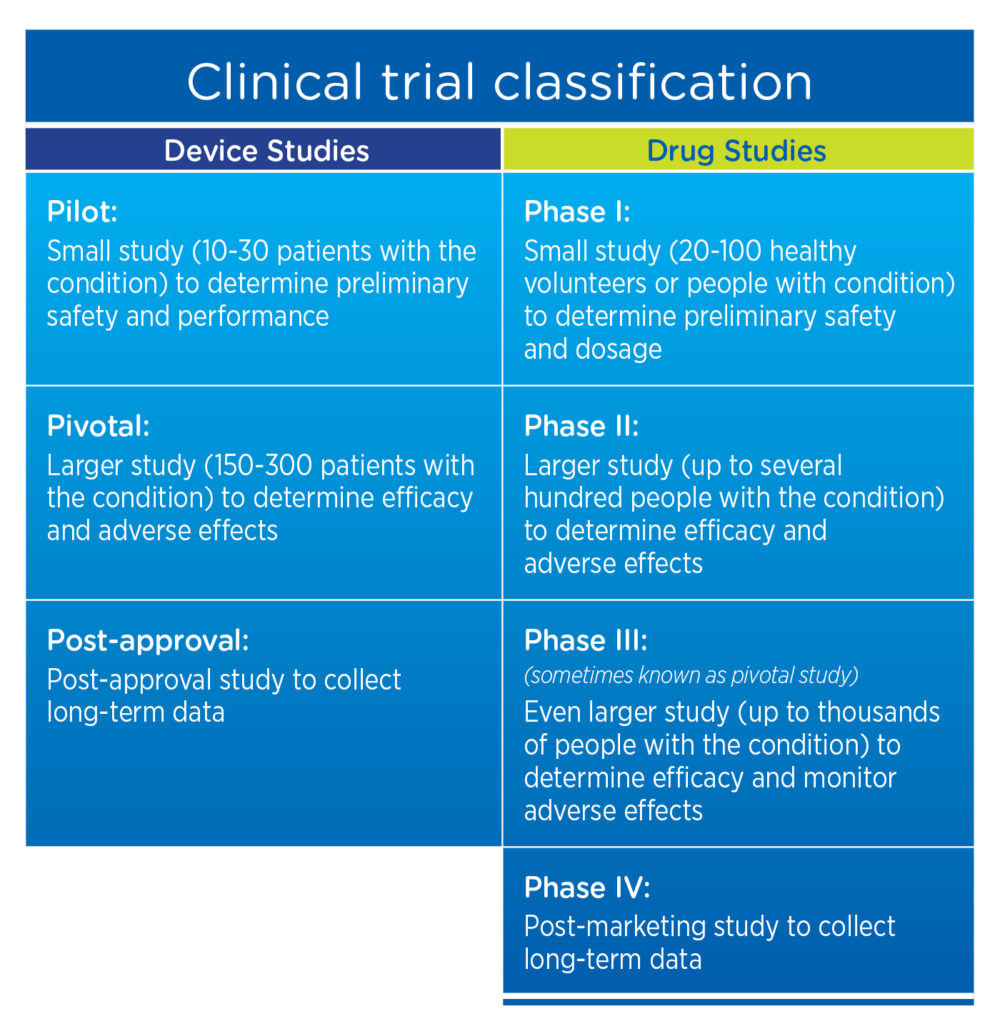
Well-designed negative trials can generate useful data, and have been published in high-impact journals (26). Trial failure means that results are not valid and so we are unable to either reject or not reject the null hypothesis. Phase II trials are also important to collect additional safety data, determining drug dosing ranges, routes and timing for phase III trials, as well as common short-term adverse events. There are numerous phase II trials evaluating the efficacy of pharmacological agents in PLP, for example, gabapentin (17), ketamine (18), memantine (19), and calcitonin combined with ketamine (20).
This is an education-orientated review of design and correct interpretation of clinical trials data. Many of the issues discussed are relevant to the correct interpretation of data from clinical trials of haematopoietic cell transplants. Quantitative endpoints should be measurable or observed on every subject in a clinical trial whereas time-to-event endpoints may not. For example, if a clinical trial collects data on subjects for 5 years after study-entry and a subject does not die during that interval the trial will not observe the time-to-death for that subject.
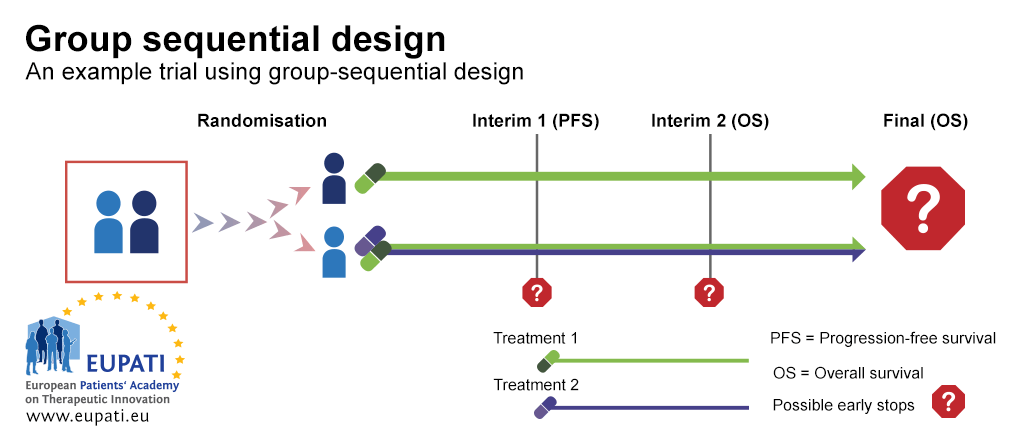
11 Planning for interim analyses
Study analysis is conducted using only data from the withdrawal phase and outcome is usually relapse of symptoms. The randomized withdrawal design aims to evaluate the optimal duration of a treatment in patients who respond to the treatment. The advantage is reduction in the time on placebo since only responders are randomized to placebo thereby giving an ethical advantage.
In this way researchers can build on models and different types of data (clinical, surrogate, etc.) from progressive trial phases, contextualizing them to improve clinical practice. In this review we introduce the underlying principles of clinical trial design, giving examples from the phantom limb pain (PLP) literature. We chose PLP given our experience designing trials for it as well as other neuropathic pain syndromes. Furthermore, PLP clinical trials have tested a variety of interventions including drugs (7,8), non-invasive brain stimulation (NIBS) (9–11), and invasive procedures (12). In addition, PLP is a disorder whose pathophysiology and optimum management remain elusive despite numerous studies.
In this study design subtype, the source of controls is usually adopted from the past, such as from medical records and published literature.1 The advantages of this study design include being cost‐effective, time saving and easily accessible. However, since this design depends on already collected data from different sources, the information obtained may not be accurate, reliable, lack uniformity and/or completeness as well. Though historically controlled studies maybe easier to conduct, the disadvantages will need to be taken into account while designing a study.
Objective endpoints are generally preferred to subjective endpoints since they are less subject to bias. The most common is hypothesis testing where researchers construct a null hypothesis (often “no effect” or “no difference”) that is assumed to be true and evidence is sought to disprove it. An alternative hypothesis (the statement that is desired to be claimed) is also constructed (often the presence of an effect or difference between groups).
This can be avoided if a controlled study design is chosen which includes a group that does not receive the intervention (control group) and a group that receives the intervention (intervention/experiment group), and therefore provide a more accurate and valid conclusion. Some of the biases observed with cohort studies include selection bias and information bias. Some individuals who have the exposure may refuse to participate in the study or would be lost to follow‐up, and in those instances, it becomes difficult to interpret the association between an exposure and outcome. Also, if the information is inaccurate when past records are used to evaluate for exposure status, then again, the association between the exposure and outcome becomes difficult to interpret. As a pioneer in the discovery and development of gene therapies for rare diseases, Genethon is a non-profit laboratory that was established by AFM-Telethon.
Comments
Post a Comment